Evidence That Early Alzheimer’s Can Be Reversed
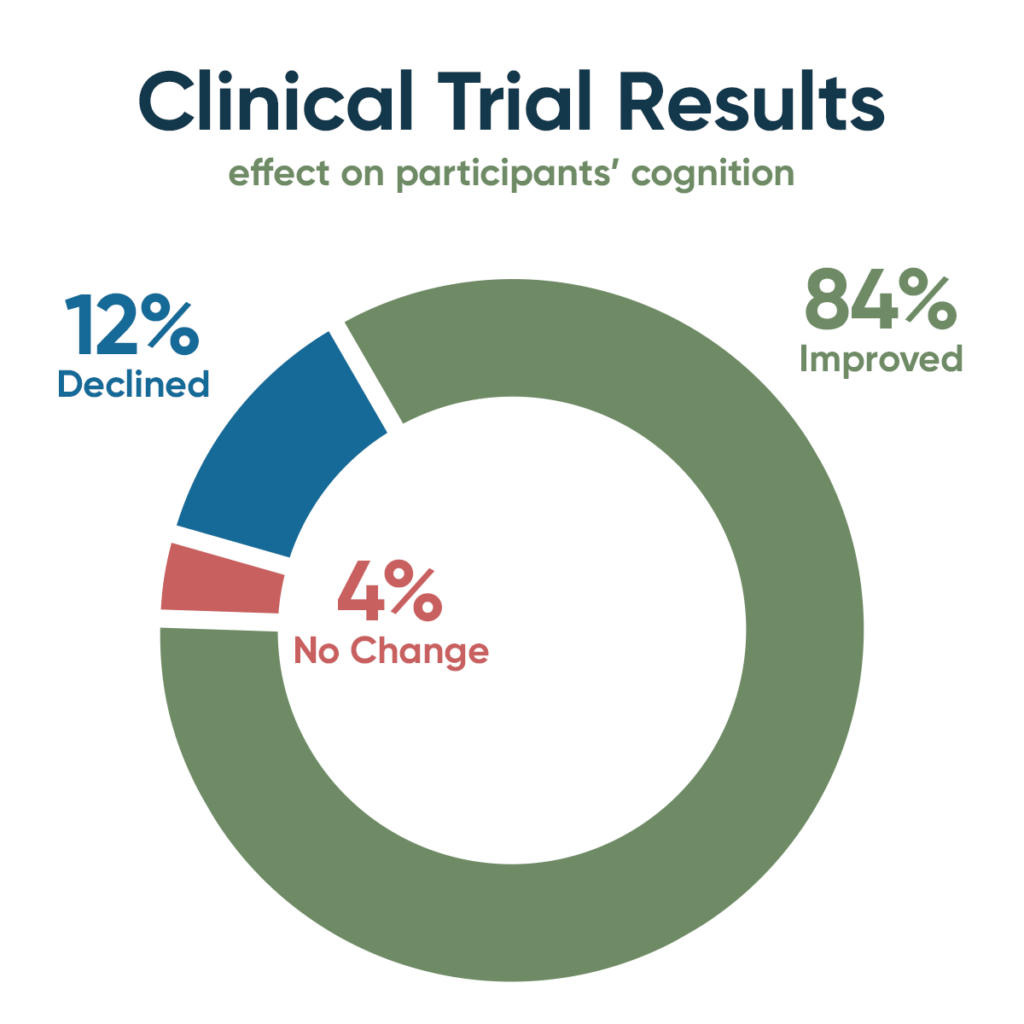
Pharmaceutical trials for Alzheimer's have seen repeated failures, but a groundbreaking study led by Dr. Dale Bredesen presents the first successful clinical trial using precision medicine to target Alzheimer's causes. Despite over 400 unsuccessful trials and minor successes with temporary improvement, neurodegenerative diseases like Alzheimer's remain a medical challenge.
Bredesen's team conducted a proof-of-concept trial with 25 participants, aged 50 to 76, all with early-stage dementia or pre-Alzheimer's. The trial assessed multiple potential contributors like inflammation, insulin resistance, nutrient and hormonal deficiencies, specific pathogens, toxins, and genetics. Each participant then received a personalized nine-month treatment. The approach aligns with that of Apollo Health, which has developed software and programs to optimize the precision medicine protocol for cognitive decline. This trial is the first prospective clinical one after numerous anecdotal case study improvements.
Trial Discussion
Please watch the videos below for important discussions regarding our successful clinical trials
This first video shows Dr. Bredesen and Apollo Health’s Chief Health Liaison, Julie Gregory, meeting with Dr. Kat Toups to discuss the recently published study.
In this video, Apollo Health’s Chief Health Liaison, Julie Gregory, Dr. Dale E. Bredesen and Chris Coward, VP of Coaching for Apollo Health discuss The First Clinical Trial to Reverse Cognitive Decline.
Dr. Dale Bredesen discusses the precision medicine trial for Alzeimer's and the trial results.